Please fill out the details below, and one of our executives will be in touch with you shortly!
CertEase is one of the top GDP Consultant in Luxembourg for providing GDP Certification in Luxembourg & other major cities such as Luxembourg City, Esch-sur-Alzette, Differdange, Dudelange, Ettelbruck, Diekirch, Wiltz. At CertEase, we understand the unique requirements of each customer & provide a tailor-made solution to meet the GDP standard requirements. Our services include implementation, training, templates, documentation assistance, gap analysis, GDP registration, Audit, and other related services at an affordable cost to all companies looking to be certified under Good Distribution Practices in Luxembourg. GDP Certification in Luxembourg is important in the Pharmaceutical industry to showcase your dedication to good distributive practices and quality in every aspect of your service. It is a quality management system for warehouse and Wholesalers distributors who are into pharmaceutical distribution. Pharmaceutical Good Distribution Practices in Luxembourg help the distributors of pharmaceutical products to get their operations & the entire supply chain aligned with the GDP standards. It process starts with sourcing raw materials for the manufacturing plant till the final shipment of finished drugs reaches the end user. GDP Certification compliance in Luxembourg is a quality mark that indicates safety & includes requirements for receiving, purchasing, storage, and export of drugs suitable for human consumption.
GDP certification in Luxembourg or Good Distribution Practice certification, is a quality standard in the pharmaceutical & medical industry. Implementing GDP helps pharmaceutical products be consistently stored, transported, and handled under the right conditions as required by regulatory authorities. The standard provides guidelines & best practices for maintaining the integrity, safety, and quality of pharmaceuticals throughout the distribution chain. Companies involved in the distribution of pharmaceuticals usually look to GDP certification to showcase their commitment to quality assurance, regulatory compliance, and the safety and efficacy of the products they handle.
GDP certificate meaning – GDP standards for Good Distribution Practice is a quality standard for distributors to ensure that the medicines maintain quality and integrity throughout the supply chain & protect public health to maintain the quality of the product, preventing contamination, degradation, or damage. Secondly, to protect public health by ensuring patients receive safe and effective medications. Overall, GDP’s introduction reflects a commitment to upholding standards that improve patient outcomes and ensure pharmaceutical integrity.
Pharmaceutical companies should implement GDP (Good Distribution Practice) certification in Luxembourg because it ensures that medicines are handled, stored, and transported properly, which helps safeguard their quality and integrity throughout the entire supply chain. By meeting the requirements of GDP standards in Luxembourg, companies can reduce the risk of contamination, counterfeiting, and other hazards that could harm the effectiveness and safety of the medicines. This not only protects patients but also helps improve the reputation and trustworthiness of the brand. GDP certificate in Luxembourg showcases commitment towards quality and compliance with legal & regulatory requirements, which is very important for maintaining the highest standards in the pharmaceutical distribution industry and also improving healthcare outcomes for people worldwide.
GDP in Luxembourg is important for pharmaceutical companies to maintain the safety, quality, and efficacy of their medicines. The standard ensures that medications are handled, stored, and transported under suitable & controlled conditions which helps minimize the risk of contamination, degradation, or counterfeiting. By complying with GDP standards, companies can showcase their commitment to meeting regulatory compliance & maintaining the quality of the medicine. This certification not only helps in keeping public health safe but also plays an important role in maintaining the quality & integrity of pharmaceutical products which helps improve trust in the industry and amongst the customers. Overall, GDP certification plays a very important role in upholding & maintaining the highest standards in pharmaceutical distribution and making sure that patients receive safe and effective medications which helps improve healthcare outcomes worldwide.
Good Distribution Practices certification in Luxembourg has so many advantages for pharmaceutical companies, Below listed are some of the important benefits of GDP certification:
Improved Quality Control: GDP guidelines in Luxembourg ensure that medicines are handled, stored, and transported under strict quality control measures, which helps minimize the risk of contamination, degradation, or tampering. This helps to maintain the safety & efficacy of medicines until they reach the patients.
Safeguards Patient Health: Meeting the requirements of the GDP standards, it shows companies give importance to patient safety. GDP compliance in Luxembourg helps prevent the distribution of low-quality or substandard drugs, reducing the chances of adverse health effects or treatment failure among patients.
Regulatory Compliance: Getting GDP Compliant in Luxembourg showcases compliance with regulatory requirements set by health authorities and industry standards. This builds trust with regulators & customers and gives assurance to the stakeholders that the company operates ethically and responsibly.
Enhances Reputation: Companies with GDP quality management in Luxembourg indicate a commitment to excellence in pharmaceutical distribution. The certificate helps improve brand reputation in the pharmaceutical industry and amongst the healthcare professionals, patients, and other interested parties who use your brand. It helps increase trust & credibility
Global Market Access: GDP certification facilitates market access by demonstrating adherence to international quality standards. This helps pharmaceutical companies to expand their market reach and distribute medicines worldwide & contributing to improved healthcare access globally.
The cost of obtaining GDP Good Distribution Practice certification in Luxembourg varies depending on several factors. These include the size of the company and complexity of the business operations, the locations that are involved in your distribution network, and the certification body you choose to work with. To know the approximate cost you can drop in an inquiry with us you will receive a detailed proposal with all the deliverables and a breakdown of the cost. This expense typically covers various aspects of the GDP implementation process in Luxembourg, such as initial requirement gathering & gap assessments, internal audits, documentation assistance & review, management review meetings, and training programs for your staff. While it might seem like a huge investment upfront GDP certification is very important for ensuring that your products are handled, stored, and distributed in compliance with quality standards and regulatory requirements. It helps safeguard the integrity and safety of your products throughout the entire supply chain, Which helps improve trust & customer satisfaction. Also, it is important to compare quotes & services from different GDP certification bodies in Luxembourg and explore flexible payment options that can help manage costs effectively & choose the right GDP consulting agency in Luxembourg to provide satisfactory services.
GDP certification program in Luxembourg offers many benefits for pharmaceutical businesses. Firstly, Being GDP compliant gives assurance to customers and regulatory authorities that your pharmaceutical products are handled and distributed with utmost care and that your process meets stringent quality standard requirements. With helping improve your brand reputation it also supports creating trust in your company’s reliability and integrity.
Secondly, GDP guidelines help streamline the distribution processes & day to day-to-day operations of your facility, which will help minimize errors that are caused and improve the overall efficiency of the facility. By implementing GDP standards in your organization, you can improve the proper utilization of your resources help reduce operational costs, and increase profitability.
Lastly, GDP accreditation in Luxembourg opens doors to new opportunities & markets worldwide. It acts as a passport to get access to domestic and international markets with new customers, where meeting the requirements of regulatory compliance is very important. The GDP certification also helps when your organization is looking to partner or collaborate with distributors or other vendors, as it showcases your commitment towards quality and compliance which is very important in the pharmaceutical industry. Overall, GDP certification has many benefits is not just a regulatory requirement but an investment to grow your business & mitigate risks, and elevate your standing in the pharmaceutical industry.
GDP in Luxembourg is primarily required for the industries that are involved in handling, storing, and distributing products that are sensitive to quality, safety, and regulatory compliance. Some of the key industries that require GDP certification services in Luxembourg include:
Pharmaceutical Industry: GDP for pharmaceuticals in Luxembourg is required for companies that are involved in manufacturing, storing, and distributing pharmaceutical products, which include prescription drugs, over-the-counter medications, vaccines, and biologics.
Healthcare Industry: Facilities and organizations that handle medical devices, diagnostics, biopharmaceuticals, and other healthcare-related products need to get a certificate for Good Distribution Practice in Luxembourg
Biotechnology Industry: Companies engaged in the production, storage, and distribution of biotechnology-derived products, such as biologics, cell therapies, and gene therapies.
Cosmetics Industry: Businesses involved in the manufacturing and distribution of cosmetic and personal care products, especially those that contain active ingredients or require controlled storage conditions.
Beverages & food Industry: Certain sectors within the food and beverage industry that deal with perishable or temperature-sensitive products may require GDP certification to maintain the integrity & quality of the product throughout the supply chain.
Veterinary Medicine Industry: Companies manufacturing, storing, and distributing veterinary drugs, vaccines, and medical devices for animal health.
Chemical Industry: Some segments of the chemical industry, particularly those dealing with specialty chemicals or hazardous materials, may require GDP registration in Luxembourg to ensure safe handling and distribution practices.
Logistics company and Distribution services: Third-party distribution & logistics service-providing companies that handle products for regulated industries would require GDP attestation in Luxembourg to showcase their compliance with quality and safety standards.
These above-mentioned industries usually have specific regulatory requirements and quality standards that monitor the distribution of their products, making GDP certification very important for maintaining compliance and integrity of the product.
Good Distribution Practice (GDP) principles are guidelines that are important for pharmaceutical products to be consistently stored, transported, and handled in a manner that maintains their quality, integrity, and safety throughout the supply chain. GDP principles include developing a strong Quality Management System in Luxembourg to look after all the distribution activities, hiring trained staff who understand their roles and responsibilities, and maintaining suitable premises and equipment for storage and transportation, and documenting all distribution activities accurately. With this, GDP principles also involve implementing procedures that help to prevent mix-ups, contamination, and any damage that might occur during the operations & making sure products are transported under suitable conditions to maintain their quality, and implementing security measures to prevent unauthorized access or tampering. GDP documentation in Luxembourg also gives importance to handling customer complaints and return policies promptly and effectively, as well as conducting quality risk management to identify and mitigate potential risks. Complying with GDP principles helps ensure that pharmaceutical products are safe and in effective conditions for end users.
GDP certification typically remains valid for three years of span, depending on regulatory requirements and industry standards the validity duration of the certificate may change based upon factors such as updation in the company policy or change in the regulations. It is important for companies to renew their GDP certification after the completion of 3 years to showcase their ongoing compliance with quality standards in Luxembourg. Regular surveillance audits and reviews should be conducted on the distribution practices to continue to meet the necessary criteria for maintaining product quality, safety, and integrity throughout the supply chain. Thus, maintaining valid GDP compliance is essential for maintaining continuous improvement & best practices in pharmaceutical distribution. Bottom of Form
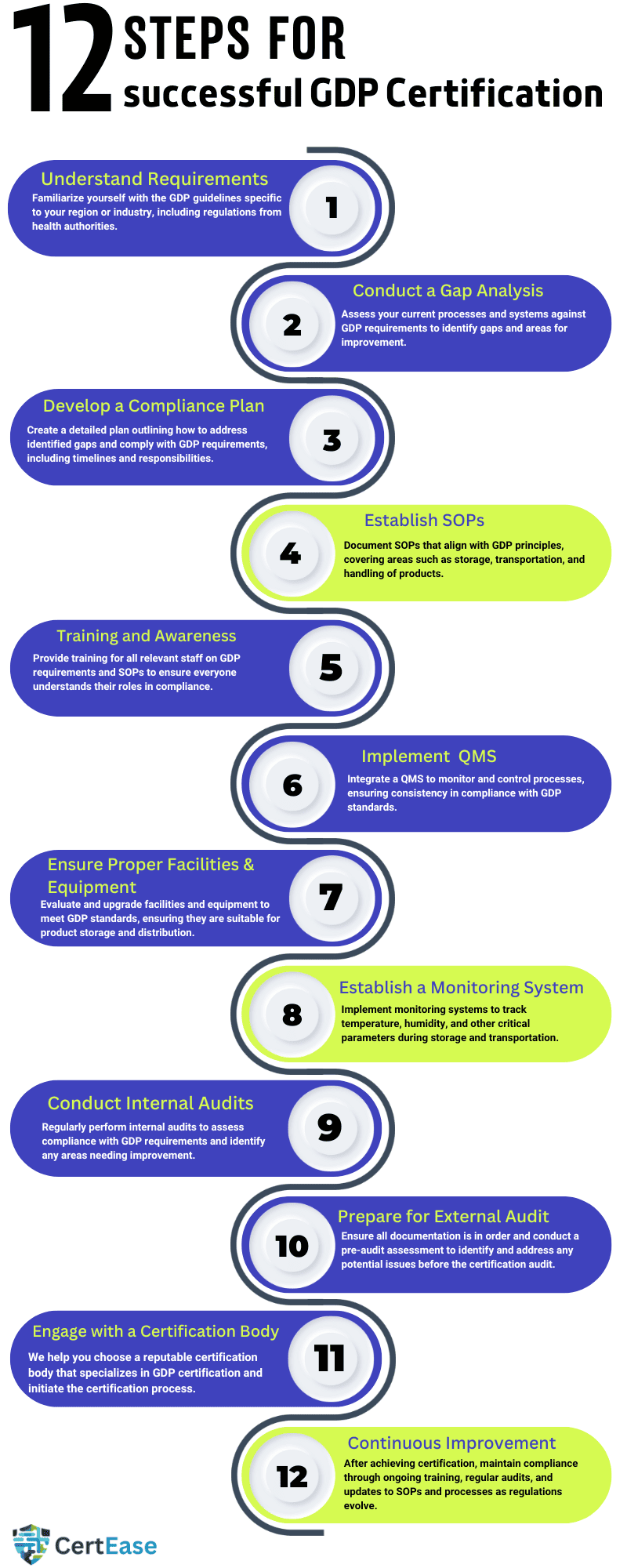
Pharmaceutical distribution certification in Luxembourg is very important in ensuring that pharmaceutical products are consistently stored, transported, and handled in a manner that maintains their quality and integrity throughout the supply chain. Here are the typical steps involved in the GDP consultation process:
Understanding the requirements of GDP: Before starting the GDP certification process, organizations must get an understanding of the specific GDP requirements relevant to their region or jurisdiction. The requirements include the regulations set by health authorities such as the FDA (in the United States), the European Medicines Agency (EMA), or other national regulatory bodies specific to the location
Assessment of Current Practices: The organization should conduct an assessment of its current distribution practices to identify any gaps or areas of improvement with GDP requirements. This involves reviewing existing standard operating procedures (SOPs), infrastructure, equipment, and personnel training programs & other relevant documentation.
Development or Revision of SOPs & other documentation: Based on the GAP assessment findings, the organization must develop or revise SOPs to ensure compliance with GDP requirements. SOPs should cover all aspects related to pharmaceutical distribution, which includes storage conditions, transportation procedures, handling practices, documentation requirements, and quality management systems.
GDP training to the staff: Personnel involved in the distribution process must undergo GDP training in Luxembourg on GDP principles, SOPs, regulations & requirements of the standard. Training programs should be done effectively and tailored to the roles and specific responsibilities of each individual within the organization.
Implementation of QMS – Quality Management System: A strong quality management system (QMS) should be implemented to oversee and monitor all aspects related to pharmaceutical distribution activities. This includes creating process documents for risk management, deviation management, change control, and continuous improvement.
Infrastructure and Compliance of the Facility: Facilities that are involved in pharmaceutical distribution must meet the requirements for storage conditions, security, cleanliness, and environmental controls. The organization checks & must make sure that its facilities comply with GDP standards by conducting regular inspections, maintenance, and by validation activities.
Record-Keeping & Documentation: Accurate and detailed documentation is very important for showcasing compliance with GDP requirements. The organization should create procedures for documenting all distribution activities which include receipt, storage, handling, and distribution of pharmaceutical products. Records must be maintained in accordance with requirements set by the regulatory bodies and should be available for inspection by regulatory authorities.
Internal Audits and Self-Assessment Audit: GDP Internal audits are conducted periodically (Once in 6 months) to check the effectiveness of the organization’s GDP processes and identify areas for improvement. Self-assessment or by hiring a GDP internal auditor organizations can evaluate the compliance with GDP requirements and check the readiness for external audits.
3rd party External Audit & assessment from the Certifying Body & Certification: Once the organization is confident about its compliance with the GDP requirements, it has to undergo an external audit by a GDP certification body in Luxembourg or the regulatory authority. The GDP auditor will check the organization’s compliance with GDP principles and other process documents such as SOPs, and other evidence related to the regulatory standards. If the organization clears the audit, then it will receive GDP certification or GDP accreditation in Luxembourg.
Continuous Improvement: GDP registration in Luxembourg is not a one-time activity but it is an ongoing commitment to maintaining the highest standards of pharmaceutical distribution. The organization must continue to regularly monitor & check and improve its GDP processes by doing regular internal audits, training, and putting corrective actions in place when needed.
By taking assistance from a GDP consultant in Luxembourg and following the above steps companies can get Pharmaceutical supply chain certification in Luxembourg and show their commitment towards the quality, safety, and efficacy of pharmaceutical products throughout the supply chain.
GDP certification requirements in Luxembourg provide the guidelines for the proper distribution of medicinal products to maintain their quality and integrity throughout the supply chain. While specific requirements may change from one to another jurisdiction, Below are some of the common requirements of GDP:
By meeting these above-listed requirements, Organisations that are involved in the distribution of medicinal products can ensure that the products that they handle, store, and transport meet the requirements of quality, safety, and efficacy throughout the supply chain.
The Good Distribution Practice audit process in Luxembourg is a thorough examination of a company’s distribution practices to check compliance with GDP quality standards in Luxembourg. It involves checking various aspects of an organization which include its storage conditions, transportation procedures, and other documentation and related evidence. During the audit, the GDP auditor in Luxembourg reviewed SOPs, infrastructure, procedure documents, and personnel qualifications to identify any areas of non-compliance or improvement. The GDP audit usually happens at the premises which includes on-site inspections, interviews with staff, and documentation reviews. Companies may receive findings and recommendations from the auditor for corrective actions for the nonconformities found during the audit. The goal of the GDP audit is to verify that pharmaceutical products are handled, stored, and transported safely and effectively throughout the supply chain. The audit process is an important measure to uphold & maintain product quality, regulatory compliance, and safety of the patients.
If you are wondering how to access GDP consulting services in Luxembourg, simply contact CertEase, one of the renowned GDP certification agencies in Luxembourg & stop solution for all your GDP certification needs, reach out to our team via email at contact@certease.com or phone at +91 8951732524 with your inquiry. Our experienced GDP consultants in Luxembourg will guide you through the process, starting with an initial assessment of your needs. Our GDP advisors will work closely with you to understand your requirements & develop a tailored plan and provide expert advice on implementing Good Distribution Practice standards in your organization. Whether you’re looking for assistance in the development of SOP, facility audits, or training the staff, Certease is here to support you every step of the way. Contact us today to ensure the integrity and quality of your pharmaceutical distribution practices and get your GDP online in Luxembourg today.

Directly or indirectly improving the organization’s profits in the short/long term in a sustainable manner
Our seasoned professionals bring expertise to every project, ensuring precision and success.
Our dedicated team ensures reliability and prompt solutions around the clock, Count on us for unwavering support.
Our experts bring verified proficiency to address your specific needs. Choose assurance, choose excellence.
Tailored to suit your specific business needs, our services make it effortless for you to obtain high-quality certifications.








Please complete the form below to receive a detailed Cost Estimation.
