Please fill out the details below, and one of our executives will be in touch with you shortly!
CertEase is the top recognized & experienced GMP Consultant in Malta, providing GMP Certification services in Malta & other major cities such as Valletta, Birkirkara, Mosta, Qormi, Sliema, Żabbar, St. Paul’s Bay. This includes services such as Implementation, Gap assessment, Documentation, Internal Audit, training & GMP Registration process in Malta at an affordable cost for all the companies & industries who are looking to get GMP complaint in Malta. GMP Stands for Good Manufacturing Practice.
GMP implementation in Malta ensures that products are consistently controlled & produced according to the quality standards. Our GMP consulting services in Malta is designed to support organizations to meet the requirements of GMP standards in Malta & help in minimize the risks associated with any production that can’t be eliminated through testing the final product. Whether you’re a small start-up or a large enterprise, CertEase is committed to helping you achieve a GMP certificate in Malta In the most efficient & economical way enabling your products to gain access to the worldwide market & improve brand reputation.
GMP also known as Good Manufacturing Practice is a set of guidelines and regulations established by the US Food and Drug Administration (FDA) to ensure that pharmaceuticals, food, cosmetics, and other consumer products are consistently produced and controlled to meet appropriate requirements of quality standards for their intended use and as required by the regulatory authorities. Implementing GMP in Malta can help organizations minimize the risk of contamination, errors, losses, and waste & helps improve brand reputation by protecting both the company and the consumer.
GMP compliance in Malta means the product is manufactured in a facility that is in accordance to the strict requirements & guidelines set by the GMP standard. Organizations getting GMP certification in Malta signify a commitment to product safety, efficacy, and compliance with legal & regulatory requirements which help improve brand reputation & customer confidence.
Different types of GMP & other relevant international standards include:
5. ISO 9001 certification in Malta: Quality Management Systems – Ensures consistent quality of products and services.
6. ISO 14001 certification in Malta: Environmental Management Systems – Addresses environmental impact and sustainability.
7. ISO 45001 certification in Malta: Occupational Health and Safety Management Systems – Focuses on workplace safety and health.
8. HACCP certification in Malta (Hazard Analysis and Critical Control Points): Ensures food safety by identifying and controlling food-related hazards.
9. ISO 22000 certification in Malta: Food Safety Management Systems – Ensures food safety throughout the food chain.
10. ISO 22716:2007 certification in Malta: Cosmetics Good Manufacturing Practices (GMP) – provides guidelines for the manufacturing, control, storage, and shipment of cosmetic products.
The cost of GMP certification in Malta can vary from one organization to another depending on numerous factors such as the size of the organization, the number of departments operating & complexity of its operations, the location of the company and the certification body which is chosen.
Generally, the GMP certification process in Malta involves expenses related to Gap assessment, documentation, training, internal audit, external auditor & consultant charges, and any updates or changes in the system or process to meet the requirements of GMP.
It’s important to consider these costs with the long-term benefits of GMP certification which include improved product quality & reliability also compliance with regulatory & legal requirements and other benefits such as customer trust, risk mitigation, and operational efficiency, and worldwide market access.
Additionally, it is important to compare quotes & services from different GMP certification bodies in Malta and explore flexible payment options that can help manage costs effectively & choose the right GMP consulting firm in Malta to provide satisfactory services.
Companies investing in GMP certification can help show their clients that are committed to producing quality products and meeting safety standards, which can lead to the growth of the organizations and more business
The “5 Ps” of GMP stand for the following key elements which help organizations comply with strict standard requirements of GMP throughout the entire production process.
People: This refers to all the individuals involved in the manufacturing process, all employees are expected to strictly adhere to manufacturing processes and understand the rules & GMP guidelines in Malta. Provide them with GMP training in Malta to make them understand their roles & responsibilities and be skilled and competent in their roles & regularly assessing their performance helps boost their productivity, efficiency, and competency
Premises & Equipment: This refers to your manufacturing facility where the production takes place & the Equipment which is used in the production. GMP regulations in Malta demand that the manufacturing facilities and equipment that are used should be designed, constructed, stored, calibrated, and maintained in a way that minimizes the risk of contamination and quality issues & is fit for the purpose of producing consistently to meet specific standards for cleanliness, environmental control, and suitability for the intended manufacturing processes.
Processes: This refers to manufacturing processes to be properly documented including the methods used to handle, process, and package products & should be communicated, distributed & understood by all employees to make sure all employees are complying with the current processes & and are meeting the required standards of the organization. Also, regular checks & evaluations are to be done to maintain consistency, reliability, and compliance with quality standards.
Products: This refers to the final product itself, it is important that products undergo constant testing, comparison, and quality assurance & checks before reaching to end user. Manufacturers should ensure that quality control measures are throughout the manufacturing process from sourcing raw materials to the finished product & all the components should have clear specifications at every phase of production so that they are produced under optimal conditions & are safe for consumer use.
Procedures: This refers to work instructions & standard operating procedures (SOPs) that govern all aspects of the manufacturing process from raw material handling to product distribution. GMP compliance in Malta requires detailed documentation to ensure consistency, traceability, and compliance of the product with GMP requirements in Malta. It must be communicated & followed by all employees and the procedures should be monitored & reviewed regularly so that there is no deviation from the standard procedure.
GMP certification is not mandatory in Malta or universally, but many countries have rules or guidelines set by regulatory authorities, industry standards, and customers that require certain industries or companies particularly those who are involved in the manufacturing of pharmaceuticals, food, cosmetics, and medical devices to adhere to Good Manufacturing Practices standard in Malta to ensure the safety, quality, and efficacy of their products.
GMP practices in Malta help create a system where products can be consistently manufactured and controlled according to quality standards suitable for their intended use.
You can get GMP certification in countries or industries where it is not mandatory, getting GMP certified in Malta helps organizations showcase their dedication to maintaining quality standards & meeting GMP requirements which can help improve market credibility, competitiveness, and consumer trust and make products stand out from their competitors.
Therefore, while GMP may not universally be mandatory, it is highly valued and often considered essential for companies that are operating in regulated industries.
If you are wondering who needs to be GMP certified in Malta, typically GMP registration in Malta is applicable & required for businesses & industries involved in the manufacturing of pharmaceuticals, food, dietary supplements, cosmetics, and medical devices. This includes manufacturers, processors, packagers, and distributors that work within these industries.
Additionally, businesses that value product quality, consumer safety, and market competitiveness may look to get GMP quality control in Malta voluntarily to showcase their commitment to quality practices and regulatory compliance.
While the specific regulations and requirements for GMP certification may vary from one country or industry to another any organization involved in the production or distribution of goods that are intended for human consumption or use can benefit from GMP certification. Thus safeguarding public health and enhancing market credibility.
There are several advantages of GMP certification in Malta that it has to offer, Below listed are some of the reasons why every organization in Malta should consider complying with GMP’s good manufacturing practices in Malta.
1. Improved Product Quality & overall performance: Good Manufacturing Practices certification in Malta ensures that products are consistently manufactured in the right conditions and controlled in accordance with the quality standards, leading to improved product quality, reliability & overall performance of the product & the facility.
2. Customer satisfaction & confidence: getting GMP accreditation in Malta helps organizations improve customer confidence by indicating commitment towards quality, safety, and reliability of products & its manufacturing processes.
3. Increased profit & Sales: Increased consumer confidence & improved operation capability help reduce waste & financial loss or fines due to product recalls, defects, or safety issues which indirectly helps increase the company’s revenue.
4. Global market access: GMP compliance certificate in Malta helps organizations demonstrate compliance with international quality standards & produce top-quality products hence enabling organizations to expand their market reach and attract international customers leading to global recognition of the brand and their product.
5. Continuous Improvement: GMP encourages a culture of continuous improvement by establishing procedures for monitoring and evaluating & by conducting regular internal audits & yearly surveillance audits helping improve manufacturing processes over time.
6. Improved operational Efficiency: GMP good manufacturing practices in Malta help streamline manufacturing processes & everyday operations leading to increased efficiency in production, reduced waste, and lower production costs.
7. Compliance with legal & Regulatory Requirements: GMP validation in Malta helps organizations meet regulatory requirements set by the local authority bodies and industry standards, and helps reduce the risk of non-compliance penalties and legal issues.
8. Awareness & commitment amongst employees: GMP standard training in Malta helps create awareness of production safety among employees & process heads which prevents numerous confusion and errors that may happen during production and also motivates employees with a sense of commitment towards the company & product safety.
1. Develop Standard Operating Procedures (SOPs) – Written procedures or flow charts for business operations or each department operating within the organization which should contain detailed instructions on how to perform specific tasks or processes in a consistent and standardized manner.
2. Implement Standard operating procedures (SOPs) and work instructions into action: This principle focuses on ensuring that tasks are carried out consistently in accordance with the established procedures or SOP’s for optimal efficiency and quality. Employees usually use shortcut methods that are different from the documented procedure. Hence, this principle encourages them to work as per the documented procedure and there is no deviation as they might not get the intended result.
3. Document procedures, processes & work outcomes: Recording work outcomes in GMP Pharmaceuticals in Malta involves documenting the results of processes & problems or complaints regarding the product to ensure compliance with quality standards and promote traceability and accountability.
4. supervise the Standard operating procedures (SOP’s) & their effectiveness: Validating SOP effectiveness ensures that standard procedures of each department & process achieve desired outcomes, maintaining quality and compliance with the requirements in GMP operations during all the stages.
5. Design and use working facilities: This principle mainly focuses on the design and development of a company’s buildings equipment and other working facilities. Implementing GMP ensures optimal conditions for manufacturing, handling, and storing products, maintaining cleanliness, safety, and efficiency standards.
6. Maintenance of equipment, facilities, and system: The main aim of this principle is to maintain the equipment and facilities properly to make sure they are functioning accurately & check product contamination to ensure that the facility or equipment is in the right condition & working accurately to avoid any kind of risks to consumers due to a lack of equipment maintenance
7. Develop skills & competence of workers on the Job: this principle focuses on each & every employee & process head that directly or indirectly impacts the quality of the product should be well trained. Basic GMP awareness in Malta related to its theory and practice should be taught, as well as role-related training should be included to improve their competency. Training should be conducted on a regular basis to keep workers updated with the new technology or changes so that they adopt & deliver high-quality products in a safe and timely way.
8. Avoid contamination by regular cleaning: This principle gives importance to maintaining proper cleanliness to prevent contamination in the product. This can be achieved by cleaning the facility on a regular basis and all the sanitation & cleaning products be followed strictly to uphold quality standards in GMP practices in Malta
9. Emphasize quality and implement it into the workflow: This principle talks about integrating quality in all phases of production & workflow, ensuring consistency, reliability, and compliance to standards in every aspect of operations. Good Manufacturing, packing, labeling, distribution, and marketing are some of the examples
10. Conduct GMP audits on a regular basis: Conduct planned GMP audits in Malta to ensure regular evaluation of manufacturing processes, facility & GMP documentation in Malta, identify areas of improvement, and maintain compliance with GMP standards for optimal performance.
1. Certification application with confirmed name, address, phone, fax, e-mail, and other details
2. Copy of company Trade & Manufacturing Licence
3. List of approved items
4. Site Master file
5. Facility Layout and equipment, instrument Records
6. Quality Manual & Quality Control Testing Records
7. Standard Operating Procedures (SOPs) & other process documents
8. List of technical staff & their qualification, experience, and approval status
9. Personnel Records
10. Batch Production Records
11. Product Specifications & Product summary sheet
12. Supplier and Vendor Qualification Records
13. Complaint Handling and Product Recall Procedures
14. Validation and Verification Documentation
15. Organisation chart
16. Water system schematic diagram
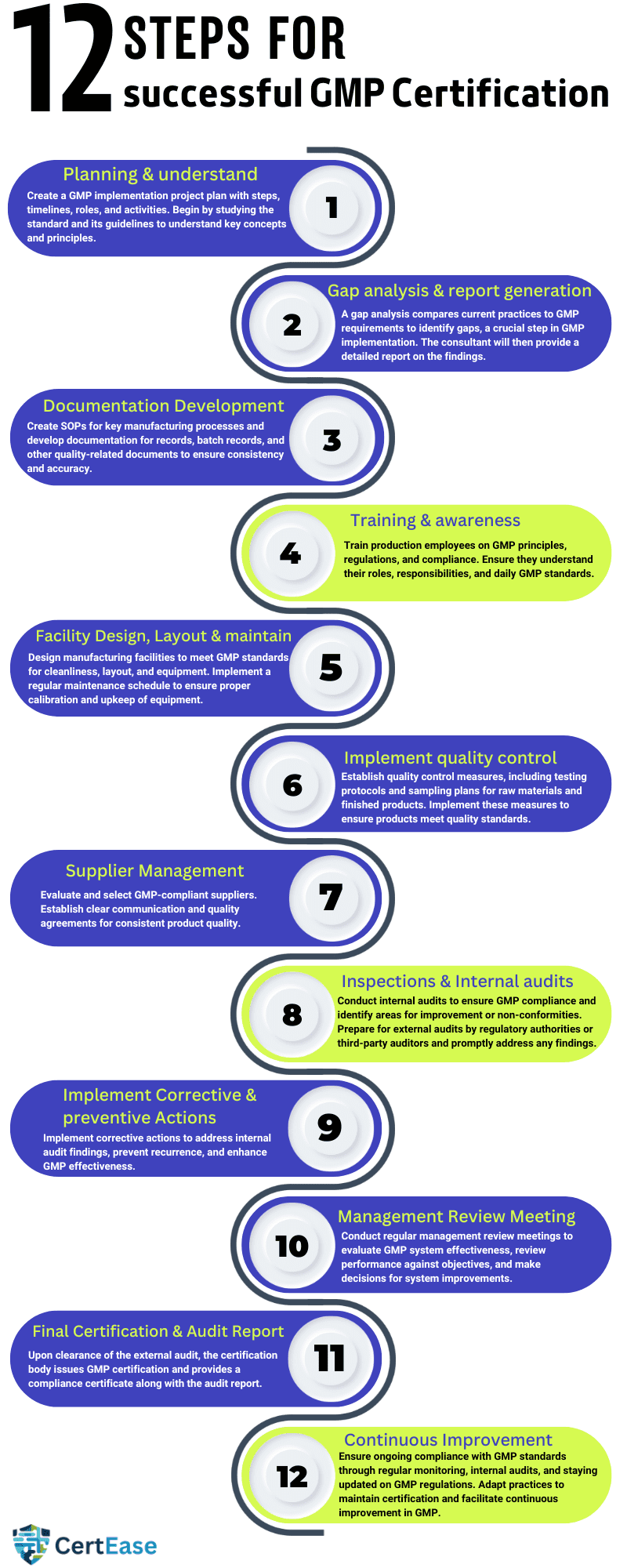
The GMP process in Malta generally involves several steps, customized in such a way as to ensure that the organization’s manufacturing processes meet all the requirements of Good Manufacturing Practices in Malta. Here’s a detailed overview:
1. Planning & understand the requirements of GMP: Develop a project plan by listing out the steps involved in the GMP implementation process, including timelines, roles & responsibilities & the activities covered throughout the journey, and understand the requirements by studying the standard and its guidelines, key concepts, principles for effective implementation of GMP standard.
2. Gap analysis & report generation: A gap analysis is a comparison of an organization’s current practices against GMP requirements & guidelines to help identify if any gaps exist in the organization’s existing practices. GAP assessment is the key step in the GMP implementation process in Malta. Based on the assessment the consultant will generate a detailed gap assessment report with the identified gaps & findings.
3. Documentation Development: Create Standard Operating Procedures (SOPs) for all important manufacturing processes Develop documentation for records, batch records, and other necessary documents related to quality to ensure consistency and accuracy.
4. Training & awareness: Provide training to all the employees who are involved in the productions and educate employees about GMP principles, regulations, and the importance of compliance. Provide training sessions to ensure all personnel understand their roles and responsibilities, dos & don’ts in maintaining GMP standards also quality & consistency in their day-to-day activity.
5. Facility Design, Layout & maintenance: Design manufacturing facilities to meet GMP requirements for cleanliness, layout, and equipment to ensure GMP compliance. Implement a regular maintenance schedule to ensure equipment is properly calibrated and maintained.
6. Implementation of Quality Control: Help the client establish quality control measures, including testing protocols, sampling plans, and specifications for raw materials and finished products. Implement sampling plans and specifications to ensure products meet quality standards.
7. Supplier Management: Do a proper Evaluation and Select suppliers based on their experience, capability & ability to meet GMP requirements. Establish clear communication channels and quality agreements with suppliers to ensure consistency in the product quality they supply.
8. Inspections & Internal audits: Conduct internal audits to check organizations’ compliance with GMP standards and identify if there are any areas of improvement & if there are any non-conformities. Prepare for external audits by regulatory authorities or third-party auditors and address any findings promptly.
9. Implement Corrective & preventive Actions: implement corrective actions to address non-conformities found in the internal audit, make necessary changes in the system to prevent the recurrence of incidents or accidents in the facility, and improve the effectiveness of the GMP.
10. Management Review meeting (MRM): conduct a regular management review meeting where top management will evaluate the effectiveness of the GMP system in Malta, review performance against objectives, and make decisions regarding system improvements.
11. Certification Audit: Schedule a certification audit with a GMP certification body in Malta. During the audit, present all the necessary evidence to the auditor to showcase compliance with GMP standards & effective implementation of the GMP within the organization
12. Follow-up after external certification audit: Address any findings or non-conformities found during the external audit. Implement corrective actions to address & resolve the issues and improve stability & compliance.
13. Final Certification & Audit Report: The certification body reviews the audit findings and decides to issue GMP certification after successful clearance of the external audit. You will receive a GMP certificate indicating your organization’s compliance with the standard along with the external audit report.
14. Continuous Improvement: Maintain ongoing compliance with GMP standards through regular monitoring, review & conducting an internal audit on a regular basis to keep up the continuous improvement of your GMP. Stay informed about updates to GMP regulations and adapt your practices accordingly to ensure ongoing certification.
GMP verification in Malta is like a stamp of approval for manufacturing facilities, indicating that they meet quality standards & produce products that are of high quality & meet safety requirements.
Usually, government regulatory agencies, such as the FDA in the US or the EMA in Europe, issue this GMP registration certificate in Malta. These bodies conduct end-to-end inspections and audits to check if the manufacturers comply with all the requirements of GMP guidelines, guaranteeing the safety and efficacy of products which include pharmaceuticals, medical devices, and certain foods.
Additionally, there are independent certification bodies accredited by these regulatory agencies that also issue GMP certifications. These bodies are specialized in checking manufacturing processes across various industries, creating trust & assurance for consumers and businesses alike.
The validity of GMP certification in Malta typically lasts for 3 years, After obtaining the initial certification, companies must undergo annual surveillance audits from the certification body to maintain their certification status.
If a company fails to meet the standards during these audits may lead to the suspension of the certification, So, it’s essential for organisation to conducted internal audits before the annual surveillance audit by the certification body to stay careful and make necessary adjustments to ensure their FDA GMP certificate in Malta remains valid and their products meet the necessary standards. Companies should renew GMP certification in Malta after completion of 3 years span.
GMP facility certification in Malta can take up to 4-6 months till the entire process is complete & you receive the final certification, the timeline also depends on factors like the complexity of manufacturing processes and facility readiness. The GMP implementation process in Malta involves many stages, which include gap assessment, training, documentation preparation, and implementation of GMP, internal audits, MRM, and finally, the certification audit by a GMP body in Malta.
Working closely with a GMP certification agency in Malta can help you understand the exact time & resources required for the entire GMP consulting & certification process and carefully following the advice of the consultant can help expedite the implementation process.
Good Manufacturing Practices certification requirements in Malta have various guidelines and the standard aims to ensure that products are consistently produced and controlled according to the quality standards relevant to their intended use. Specific requirements can change depending on the industry and regulations, below listed are some of the common elements:
1. Facility and Equipment: Proper facilities and equipment must be in place to make sure that there is quality and integrity of the manufacturing process & it is maintained as per GMP requirements, which should also include necessary maintenance and calibration procedures.
2. Personnel: Proper training and qualifications should be provided to employees who are involved in manufacturing, quality control, and other relevant processes within the facility to improve competency in their work and maintain GMP quality standards in Malta.
3. Record-Keeping & Documentation: Organisations should maintain extensive documentation of procedures, processes, and records related to manufacturing, testing, and quality control activities to showcase compliance with GMP requirements.
4. Quality Control: Strong quality control measures should be implemented & maintained throughout the manufacturing process to monitor and check the quality of raw materials till the finished product.
5. Hygiene & Sanitation: Organisations should take strict measures for maintaining cleanliness, sanitation, and hygiene in production facilities to prevent any type of contamination and ensure the safety of the product.
6. Validation & Testing: It is important for organizations to regularly do validation & checks on their manufacturing processes, testing procedures & analytical methods to maintain the quality and consistency of the product.
7. Complaint Handling & Product Recall: organization should maintain a procedure for handling complaints of the customer, product recalls & managing issues related to the quality & safety of the product.
8. Continuous Improvement: the organization should implement a system to continuously track & monitor the manufacturing processes and QMS to maintain the quality & safety of the product with GMP standards, evaluate & check if there are any areas to improve
These are just some of the key requirements for GMP certification, specific requirements may change depending on different industries and regulations. It’s very important for manufacturers to stay updated on any changes in regulations and standards to ensure compliance and maintain GMP certification.
here are the roles and responsibilities of a GMP (Good Manufacturing Practice) consultant presented in bullet points:
Generally, a GMP consultant in Malta plays an important role in helping companies achieve and maintain compliance with GMP standards and making sure they produce high-quality and safe products.
The GMP auditing process in Malta involves several key steps to check the manufacturing facility’s compliance with quality standards. GMP audit checklist in Malta involves the auditor review of documentation, which includes procedures, records, policies, and quality management systems, to ensure they meet GMP requirements.
Then, they conduct an on-site inspection, checking the facilities, equipment, and production processes to verify compliance with GMP standards. During the inspection, auditors observe operations, interview department heads & employees, and collect samples for testing.
After the inspection, the auditor collects all the findings from the audit and prepares a detailed audit report that includes observations, non-compliance, or areas for improvement. The audit report is then shared with the company for review and to implement corrective action.
Finally, based on the audit findings, the GMP certifying body in Malta makes a decision regarding the certification. If the facility meets the requirements, the certification is granted. If not, the company may need to address non-conformities and undergo an audit one more time before certification can be awarded. Overall, the GMP audit process ensures that manufacturing practices align with GMP standard requirements to justify product quality and safety.
If you are wondering how to get a good manufacturing practices GMP certificate in Malta for your company, CertEase, one of the well-recognized GMP certification providers in Malta is the one-stop solution for all your GMP consulting & certification needs, You can reach out via phone at +91-89517-32524 or email us at contact@certease.com. Our dedicated team is here to support you every step of the process, helping your manufacturing practices align with the highest standards & quality GMP compliance.
With our expertise and guidance, we help you navigate the GMP certification online in Malta journey seamlessly, helping display your commitment to producing safe and reliable products. Whether you need any assistance have any questions related to the process, documentation, or require on-site support, we’re here to guide you. Contact us today to start the conversation and work together to achieve GMP certification for your business in Malta.

Directly or indirectly improving the organization’s profits in the short/long term in a sustainable manner
Our seasoned professionals bring expertise to every project, ensuring precision and success.
Our dedicated team ensures reliability and prompt solutions around the clock, Count on us for unwavering support.
Our experts bring verified proficiency to address your specific needs. Choose assurance, choose excellence.
Tailored to suit your specific business needs, our services make it effortless for you to obtain high-quality certifications.








Please complete the form below to receive a detailed Cost Estimation.
